INTRODUCTION
Bengal delta plain (BDP), an integral part of the world's largest delta, is a major natural storehouse of high As groundwater where millions of people are now suffering fromserious health hazards including arsenic induced cancer (Guha Mazumder et al., 1999).The unconsolidated alluvium of Pleistocene-Recent age is the source horizon that restsupon Tertiary rock sequence (Bhattacharya et al., 1997).
The scale of the problem is grave and unprecedented both in terms of human exposure(~ 60-80 million) and geographical area coverage (173x 103 km2) (PHED, 1993; Bhattacharya et al., 1997; Smedley and Kinniburgh, 2002; Bhattacharya et al., 2003). The presence of As in groundwater, higher than the stipulated Indian standard (50 μg L-1, drinking water quality standard for most countries) and WHO guideline value (10 μg L-1) for human consumption was first reported from West Bengal, India during early 1980’s with first diagnosed case of arsenicosis (Saha, 1984). Later on, within a span of decade, human suffering from natural dissolved inorganic As was widespread in the West Bengal part of BDP and with frequent cases of arsenicosis was reported from 72 blocks spread over nine districts covering ~ 3700 km2 in West Bengal, starting from Malda in the north to the 24- Pargana (s) in the south (Bhattacharya et al., 1997; Bhattacharya, 2001). In west Bengal high As groundwater areas (barring Purbasthali, Bardhaman district and Balaghar, Hugli district) are confined to the west of Bhagirathi River. However, in the eastward extension of BDP (Bangladesh), the natural arsenic “hot spots” have much wider spatial distribution In those ’hot spots” more than 90% of the shallow wells are shown to have elevated levels of arsenic compared to BangladeshNational Standard (50 μg L-1), though the resultant health problem was first diagnosed only in 1993 (BGS and DPHE, 2001; Smedley and Kinniburgh, 2002; Ahmed et al., 2004).
In nature, arsenic (0, -3, +3, +5 oxidation states) exists in both inorganic as well asorganic form. Dissolved forms of arsenic in the natural water includes arsenate (+5),arsenite (+3), mono-methyl arsonic acid (MMA) and di-methyl arsinic acid (DMA).However, the degree of toxicity solely depends on the form (inorganic/organic) and theoxidation state of the element. The inorganic forms (arsenate/arsenite) are more toxic than organic forms (MMA/DMA). Among the inorganic forms, the trivalent form (AsO33-) are likely to be more toxic than pentavalent ones (AsO43-). Therefore, the precise concentration (chemical speciation) and chemical forms of dissolved inorganic arsenic (both aqueous and solid phases) are important because more toxic and labile As (III) is now globally identified as a major public health issue.
The understanding of the behavior and distribution of redox species in natural watersare essential to explain recently found large-scale groundwater contamination reportedfrom various part of the world. Therefore, the present paper focuses on thehydrochemistry of high As groundwater as well as interaction between water andarsenic traps to understand the release of redox sensitive species into groundwaterunder local reducing condition (orthodox redox traps). Moreover, understanding thedistribution of redox species in natural waters is also essential to explain the large-scalegroundwater contamination in the BDP. The different forms of As (in both aqueous andsolid phase) will also be identified to assess their roles in As mobilization as well as levelof toxicity during human exposure. Attempts have also been made to visualize thegeochemistry of the deltaic environment in relation with speciation of As.
GEOLOGY AND PHYSIOGRAPHY OF THE BENGAL DELTA PLAIN
The Bengal basin is a large asymmetrical pericratonic basin and located in the northeasternpart of the Indian sub-continent and one of the largest sedimentary basins ofthe world. The BDP is an integral part of this basin, with an accumulation of fluviodeltaicto deltaic-estuarine sediment. The West Bengal part is characterized by a longdepositional history of sedimentation (Mesozoic to Recent) that was deposited on aPrecambrian basement. The zone is demarked by subsurface domal structures ofvarying dimension bordering east by a row of enechelon faults. The BDP hascharacteristic topography, geomorphic and geologic features (through space and time)within the sediments. Throughout the Holocene period, the BDP seems to have acted asfluvial-estuarine-marine platform where both marine (lower part of delta) and nonmarine(upper delta and valley margin fan) sedimentation took place. The BDP sedimentconstitutes dissected uplands with rolling topography and high alluvial plain above theregime of the present day river system. These sediments often disconformably overliethe older deposits, parts of which have been configured as low-lying swamp providingbase for tidal inlets beyond the level of inundation of the rising sea-level at thebeginning of the Holocene. Towards end of the mid-Holocene, the sea level has retreatedto the south of Ranaghat-Khulna axis of the delta. The large inland swamp areas areoften associated with vegetation cover, that could possibly be the source of SedimentaryOrganic Carbon (SOC) that may have played as an active agent of metal deposition andisolation (Chatterjee et al., 2004).
The Bangladesh part of BDP, located at the head of the Bay of Bengal, occupies most ofthe Bengal basin. The basin is surrounded by the Indo-Burman range in the east, anuplifted block of Precambrian Shield (Shillong Plateau) in the north, and Precambrianbasement complex (Indian Shield) in the west. More than 16 km thick syn-orogenicCenozoic sediments are deposited in the basin derived from the Himalayan and Indo-Burman range (Uddin and Lundberg, 1998). Tertiary sediments in Bangladesh arerepresented mainly by sandstone and shale sequences, while Pleistocene sediments arerepresented mostly by clay, overlain by Holocene alluvium.
Various geomorphological units were mapped in the BDP (Morgan and McIntire, 1959;Umitsu, 1987; Brammer, 1996), which include piedmont plains, flood plains, deltaplains and coastal plains. The geomorphological units in the Holocene landmasseswithin the Bangladesh part of delta include fan, flood plains, the moribund delta, the Chandina plain and upland Pleistocene Terraces (Barind and Madhapur Tracts) (BGSand DPHE, 2001).
STUDY AREA
 The study area (Nadia district, ~ 65 km north of Calcutta, West Bengal, India, Latitude 23oN and Longitude 88oE) forms an integral part of the Ganga- Brahmaputra-Meghna (GBM) fluviodeltaic system (Figure 1). The vast alluvium plain spreading from Karimpur (north-east of Calcutta) down to the Bay of Bengal is characterized by succession of a fining upward sequence with occasional clay layers. The fluviatile-estuarine deposits has been influenced by grain size variation (reworked as well as distributed), mineral deposition, organic matter etc. Fluvio-marine deposits are also present in the near surface with organic matter and variable thickness of clay lenses are often observed in such environment. Overall formations are complex and interfingering in nature.
The study area (Nadia district, ~ 65 km north of Calcutta, West Bengal, India, Latitude 23oN and Longitude 88oE) forms an integral part of the Ganga- Brahmaputra-Meghna (GBM) fluviodeltaic system (Figure 1). The vast alluvium plain spreading from Karimpur (north-east of Calcutta) down to the Bay of Bengal is characterized by succession of a fining upward sequence with occasional clay layers. The fluviatile-estuarine deposits has been influenced by grain size variation (reworked as well as distributed), mineral deposition, organic matter etc. Fluvio-marine deposits are also present in the near surface with organic matter and variable thickness of clay lenses are often observed in such environment. Overall formations are complex and interfingering in nature.The geomorphology is characterized by a series of meander scars of varying wavelengthand amplitude, abandoned channels, ox-bow lakes etc. Common land form features arenatural levees, back swamps and inter-distributory swamps. The area has a naturalsouthward slope with a relief difference of few meters (~ 2-5 m). The climate is tropical,hot and humid (temperature range 16-42oC, average relative humidity > 65%) with annual rainfall ranging between 1295-3945 mm (mostly concentrated during themonsoon, June – October).
The Bangladesh study area (Figure 1) is different from the West Bengal part ingeomorphological aspects because Lower Delta Plain (LDP) was severely dissected indrainage pattern as well as relief. Climatically, Bangladesh is not much varied fromWest Bengal except heavy monsoon and high humidity. Groundwater development isalmost similar to West Bengal. However, exploitation of shallow aquifers for irrigationare in much larger scale to sustain agriculture. Quaternary sediments provide goodaquifers in West Bengal and Bangladesh but As-enrichment is mainly restricted to theHolocene alluvial aquifers at shallow and intermediate depths (Guha Mazumder et al.,1999; BGS and DPHE, 2001; Ahmed et al., 2001; Bhattacharya et al., 2002a).Quaternary sedimentation in the BDP is largely controlled by huge sediment supply,active tectonics and sea level changes (Goodbred and Kuehl, 2000).
METHODS
Sampling (both aqueous and solid phase)
Groundwaters were sampled from existing domestic tubewells in nine As affecteddistricts of Bangladesh during January 1999 and 2001. The location of each tube wellwas determined using a hand held global positioning system (GPS). Groundwater pH,redox potential (Eh), temperature and electrical conductivity were measured in the field.pH was measured using a Radiometer Copenhagen PHM 80 instrument using acombination electrode (pH C2401-7). Redox potential was measured in a flow-through cell using a combined platinum electrode (MC408Pt) equipped with a calomel referencecell. Water samples collected for analyses included: i) filtered (using Sartorius 0.45 μmonline filters) for anion analyses; ii) filtered and acidified with suprapure HNO3 (14 M) for the analyses of cations and other trace elements including arsenic (Bhattacharya et al., 2002b). Arsenic speciation was performed with disposable cartridges® (MetalSoft Center, PA) in the field, following the methodology described by Meng et al. (1998). The cartridges, strongly adsorbs As(V) while allowing As(III) to pass through. Similarprotocols and methodology were practiced during groundwater sampling from numberof affected villages of Nadia, West Bengal.
Bulk sediment was collected from various boreholes drilled during field investigation.Sediment samples were sealed in polythene bags under inert atmosphere and thenstored in an airtight ice-cooled box (temp ~ 4oC). Details of sampling protocols,measurement techniques for both water and borehole sediment has been described inprevious publications (Bhattacharya et al., 2003).
Analysis (both aqueous and solid phase)Anions were analyzed by using ion chromatograph (Dionex 120/Metrohm 761),simultaneously Tecator AQUATEC 5400 analyzer was used to measure NO3- (540 ηm) and PO43- (690 ηm). Cation analyses were done by ICP-MS (Varian/Jobin-Yvon/Perkin- Elmer). Arsenic [Astot and As(III)] were determined using AAS (Perkin-Elmer) and a few bulk sediment samples were also analyzed by ICP-AES (Perkin-Elmer). Redox sensitive species were also analyzed spectrophotometrically (Perkin-Elmer, Lambda-20). During the measurement, ultra trace elements [mostly As(III)] were diluted in various ratios depending on their concentration and buffered (0.5M citrate buffer) for their selective measurement under controlled condition. Measured As(III) was also verified with disposable cartridge separation method and found that there was no significant difference in As(III) concentration (~ 5%). Dissolved organic carbon (DOC) in the water samples was determined on a Shimadzu 5000 TOC analyzer (0.5 mg L-1 detection limit with a precision of ±5 -10%). Certified standards, SLRS-4 (National Research Council, Canada) and GRUMO 3A (VKI, Denmark) and synthetic chemical standards prepared in the laboratory, and duplicates were analyzed after every 10 samples during each run. Trace element concentrations in standards were within 90-110% of their true values. Relative percent difference between the original and duplicate samples were within ±5 - 10%.
Chemical partitionings, using acid (7M HNO3), oxalate (0.2M NH4-oxalate, pH~3.5) and buffer (Na-acetate, pH~5.4), were performed in finer sediments (tot and associated elements (Fe, Mn, Al, P and S). Crystalline phases were also identified using an X-ray Diffractometer (Philips). Magnetic and non-magnetic fractionare separated by using a hand magnet. Sediments from different depths and individualmineral phases separated from each sample were then treated for As and associatedelements analysis by using AAS. Organic carbon of the air dried borehole sedimentsamples are determined as discussed in Bhattacharrya et al. (2003).
RESULTS AND DISCUSSION
Groundwater chemistry and behavior of arsenic
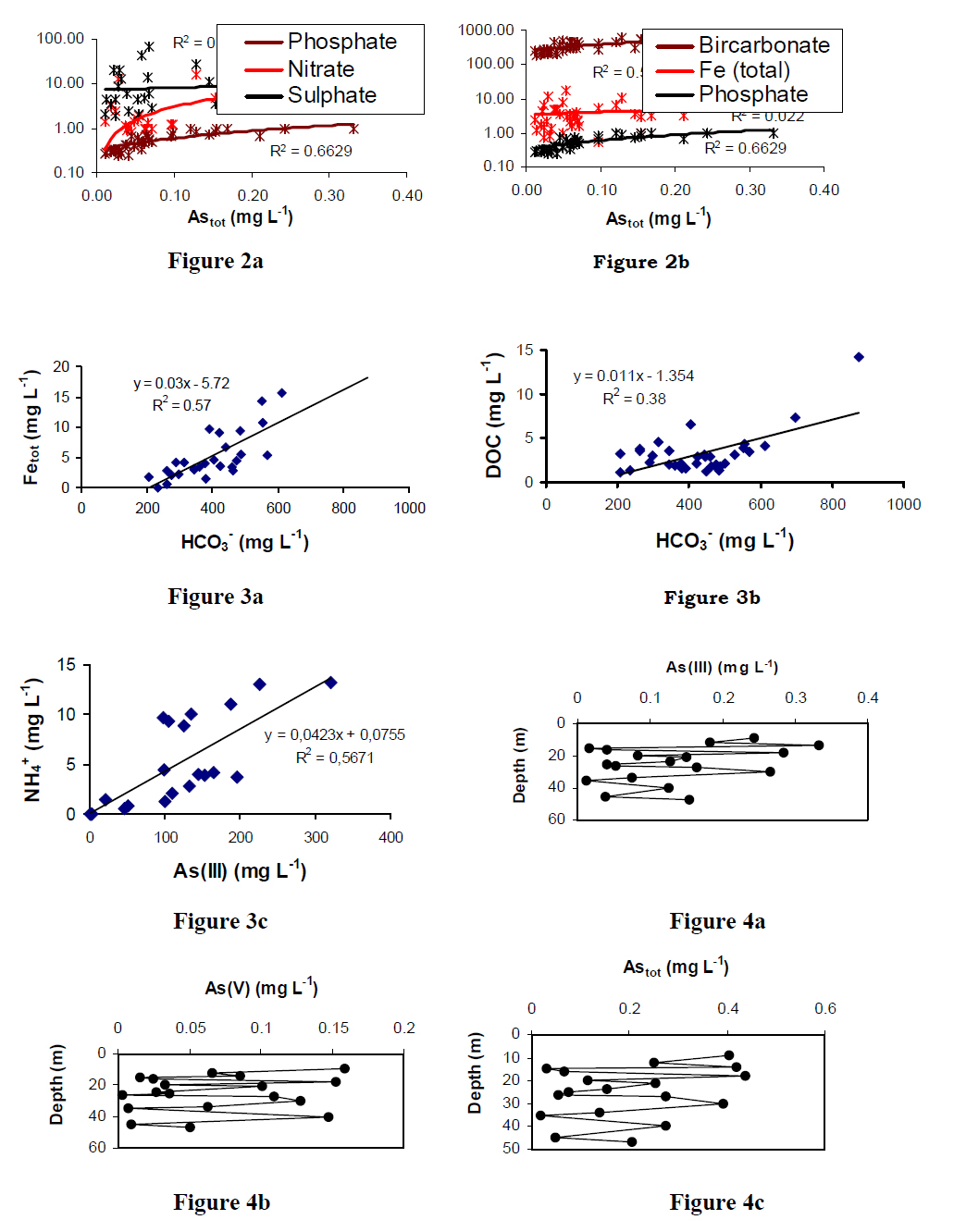
Most groundwater are of Ca-/Ca-Mg- HCO3 type, although Ca-Na-/Na-Ca- HCO3- type are also found in the saline tracts of Bangladesh where Cl- concentration goes up to ~ 4000 mg L-1. The characteristics chemical features of the natural high As groundwater of BDP (both West Bengal and Bangladesh) are low to very low dissolved oxygen (-1), high redox-sensitive metal species (Mn > 0.4 mg L-1, Astot > 1 μg L-1, Fe > 0.2 mg L-1), HCO3- (> 300 mg L-1), PO43- (> 0.6 mg L-1) and DOC (> 2 mg L-1) and with low Eh (generally 3- (-1), SO42- (-1) and nearly neutral pH (6.5-7.5). Therefore, the characteristic features of groundwater from BDP clearly demonstrate the typical anoxic nature of the aquifers (mostly shallow/intermediate). Major ion composition is HCO3- (300-620 mg L-1) that varies with depth and lithology. Distribution of major anions (NO3-, SO42-, PO43-) indicates low variability, while mapping of groundwater PO43- indicates that the high groundwater PO43- areas are also arsenical (Figure 2a). Distribution of the major cations (Ca2+ ~ 21-184 mg L-1, Mg2+ ~ 14-96 mg L-1, Na+ ~ 7-170 mg L-1 and K+ ~ 1.5-20 mg L-1) showed significant aerial variations with depth. Results of chemical analyses also showed large variation in the concentration of metal redox species [Astot ~ 2.5-1020 μg L-1, As (III) ~ 6-970 μg L-1, Fetot ~ 0.2-15.7 mg L-1, Fe (II) ~ 0.2-15.3 mg L-1], thus indicating the presence of elevated levels of both As and Fe in groundwater. Arsenic (Astot) concentration in the groundwater varies over 3-4 orders of magnitude in some of the shallow wells, and frequently exceeding the WHO guideline value. As concentrations showed little distinct regional trend with other measured water quality parameters and exhibits a significant short-range spatial variability. Speciation data indicate that the ratio of As(III)/(V) is varying largely over a large geographical area. However, the distribution of Astot and As (III)/(V) ratio in the groundwater is largely fluctuating. There is a tendency of high Astot and As(III) concentration (> 210 μg L-1) of wells located in the low-laying areas (local sagging zones).
There is a positive correlation between Astot, PO43- and HCO3- whereas the correlation with Fe is not significant (Figure 2b). On the other hand in Bangladesh, Fetot indicates positive correlation with HCO3- (Figure 3a). This is commonly expected in BDPgroundwater with reducing environment where dissolved Fe(II) concentration is largelycontrolled through precipitation/co-precipitation of iron carbonate/phosphate (e.g.,siderite, vivianite etc.). The high alkalinity load in the groundwater is due to thebreakdown of fresh organic matter during the activity of microbes that has beenregulating the process. Groundwater temperature is relatively high (26-31oC) and elevated groundwater temperature further facilitating the microbial process that leadsto increase in the local reducing condition. Partial pressure of CO2 is also high in contaminated groundwater (log pCO2 ~ 1-2.5) and calcite, dolomite, siderite and vivianite has been found in supersaturated phase. Hydrogeochemical model (Parkhurst,1995), suggests that the dissolved Astot and Fetot concentrations is depending on the dissolutions/precipitations of above mineral phases, therefore, it is unlikely to have a strong positive correlation between As and Fe in groundwater. On the other hand, elevated HCO3- levels are not only controlled by the dissolution of carbonates, where HCO3-concentrations shows overall positive correlation with DOC (Figure 3b). Thus favouring the breakdown of organic matter, and is important in controlling the thermodynamically favoured microbial reactions. Both SO42- and NO3- does not showany correlation with Astot and are generally in the low to very low concentration range in groundwater. Low NO3- concentration in groundwater is mainly due to consumption during microbial process before Fe reduction (de-nitrification process). There is a moderate to strong correlation between As(III) and NH4+ (r2 = 0.57, Figure 3c) in Bangladesh groundwater where local reducing environments are more predominant and is observed because nitrogen (breakdown product of nitrification process) can be further reduced to NH4+ under strongly reducing condition. Therefore, such areas frequently show the presence of more toxic/labile As(III) in groundwater. This could be the reason of large number of patients have been identified from BDP hot spot areas (Guha Mazumder et al., 1999; Karim, 2000).
Distribution of arsenic species
Groundwater As problem is serious in terms of both geographic distribution (discretesource) and scale of exposure (exposure risk from health point of view ) even if withinsafe limits i.e. low As (tot exhibits a depth coverage of much wider areas (Figure 4c).Therefore, in many areas it appears that the tubewells having depth greater than 150 m(deep aquifer) can only provide low As water. The increasing concentration of As (III) isalarming due to the increased recognition of the significant outbreak of As inducedcancers and internal health problems in BDP resulting from the extensive use ofshallow groundwater which contains largely As(III) compared to As(V) (Bhattacharya etal., 1997). Therefore, aquifer mapping is essential to decipher the high- and low-Aszones to ensure the level of As(III) in groundwater.
Sediment chemistry
The distribution of As (Astot ~ 10-26.3 mg kg-1), organic matter (SOCtot ~ 4.2-9.5g kg-1) and iron (Fetot ~ 0.5-1.6 g kg-1 ) demonstrates that the fine grained sediments (silt/silty clay) were found to be associated with high groundwater As areas. A close examination of the chemical characteristic of sediment further reveals that there is a positive correlation (r2 = 0.67) between elemental As and Fe, although the elemental As is absent in the structure of arsenic traps. On the other hand, there is a weak correlations (r2 = 0.22) between elemental As and C, where average C content of the sandy sediment is often less as compared to silty/silty clay horizons.
Acid oxalate extractable fractions of Feox, Mnox, Alox, Pox and Asox also revealconsiderable variability with depth and lithology. The range of Feox, Mnox, Alox, Pox andAsox varied between 0.4-5.9 g kg-1, 0.005-0.45 g kg-1, 0.1-1.32 g kg-1, 5.1-144 mg kg-1and 0.1-8.6 mg kg-1, respectively. A positive correlation was observed amongst Asox and Feox, Mnox as well as Alox (Figure 5a-c). While the coarse-grained sediments indicated strong positive correlations between Asox and Pox (Figure 5d), their distribution in thefine-grained sediments (silty clay and clay) revealed an inverse trend (Figure 5d).Distinct negative correlation between Asox and Pox fractions suggests dissolution of secondary vivianite, rather than the release of PO4 ions adsorbed onto the Fe-oxide surfaces in these fine grained sediments. Acetate extractable fractions Feacet (0.02-0.37 mg kg-1), Mnacet (0.002-0.27 mg kg-1) and Pacet (0.7-73.7 mg kg-1), account for a range of 2.1-80% Feox, 15.8-97% Mnox, and up to 70% Pox. Dissolution of minerals like siderite and rhodochrosite may account for the acetate extractable fractions of Feacet and Mnacet in these sediments. However, high acetate extractable P fractions (Pacet) concomitantwith high Fe (Feacet) indicate the possible dissolution of vivianite in these reducing aquifer sediments (Dodd et al., 2000). It is important to note that the hydrogeochemical speciation modeling also suggests that the groundwater are supersaturated with respect to these minerals, which act as sinks for Fe and Man as well as PO43- and hence control their solubility in groundwater (Bhattacharya et al., 1998; Nickson et al., 2000). Oxalate extraction of the aquifer sediments demonstrate that the As and other associated elements (Fe , Mn , Al , P) were dominated the solid phase (Feox ~ 0.2 -5.9 g kg-1, Mnox~ 0.005-0.45 g kg-1, Alox ~ 0.02-1.32 g kg-1, Pox ~ 5.1-144 mg kg-1, Asox ~ 12-101 mgkg-1), and the significant variability was observed with depth, topography, geomorphic and geologic features. It has also been observed that Asox and Pox showed positive correlation (Figure 5d) in coarser sediments rather than their distribution in the finegrained sediments.
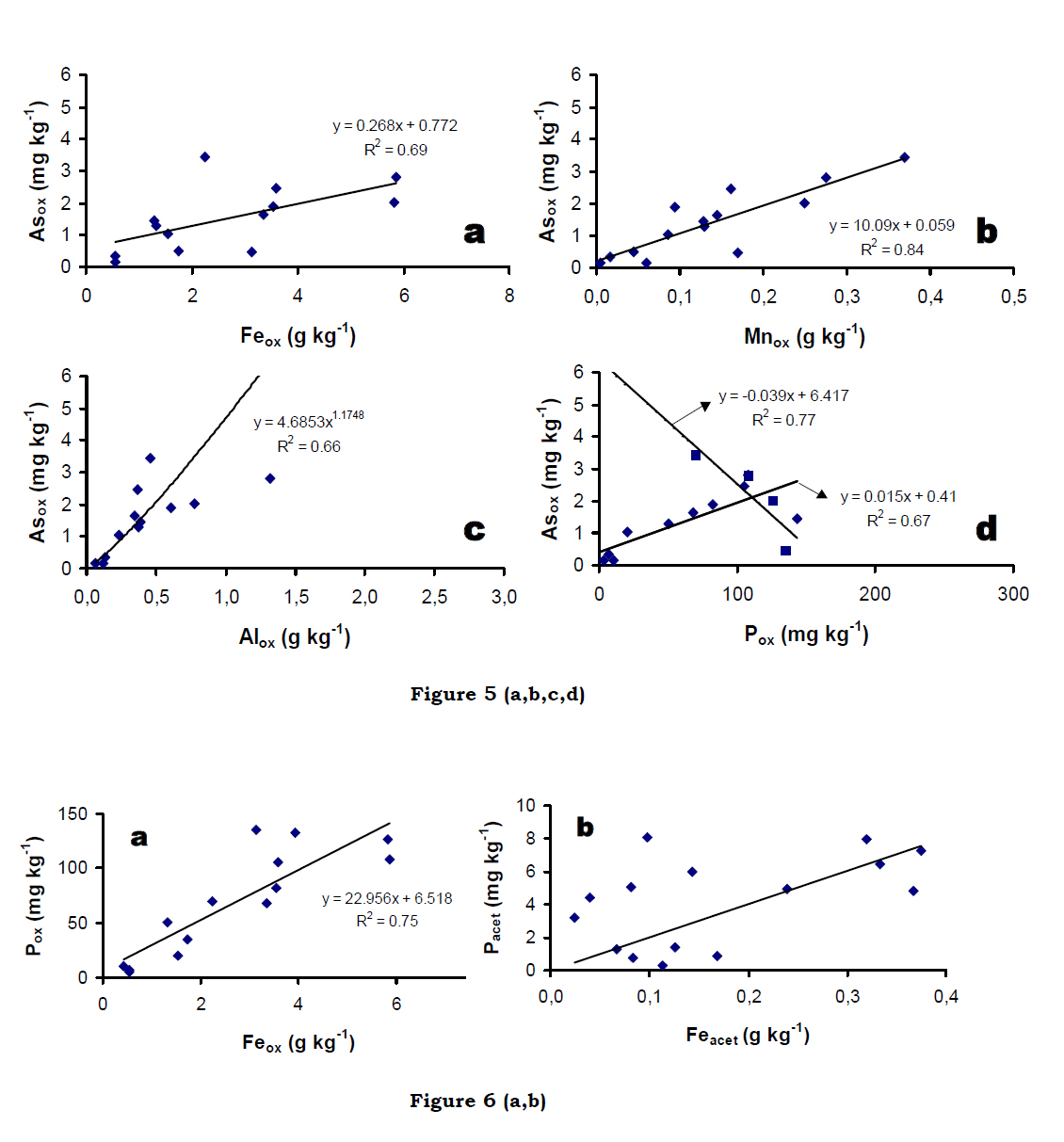 Furthermore relatively high acetate fraction (Feacet ~ 0.02 – 0.37 mg kg-1 in Feox ~ 2.1 – 80%, Mnacet ~ 0.002 – 0.27 mg kg-1 in Mnox ~ 15.8 –97%, Pacet ~ 0.7 – 73.7 mg kg-1 in Pox ~ 70 %) confirms the possible dissolution of carbonate (siderire and rhodochrosite) as well as unstable phosphate mineral (vivianite) that has already been demonstrated by our speciation model during aqueous phase discussion. The study (hydrogeochemical and chemical partitioning modeling) further suggests that thegroundwater were supersaturated with respect to carbonate and phosphate mineralswhich acted as sinks for Fe/Mn/PO43-, and thereby, control their solubility in groundwater. Carbonate minerals were important components of mineralogy of the BDP sediments and dissolution/precipitation of As from such mineral phases controlsgroundwater chemistry. Therefore, reaction kinetics are important in understanding thelocal short range spatial variations. Moreover, correlation between Feox and Pox (r2 = 0.74, Figure 6a) indicates reductive dissolution of the amorphous Fe–oxides together with surface bound PO43-, whereas Feacet and PO43-acet fraction do not exhibit such relationship. These suggest that the Fe-oxides – PO43- pool is more significant thanvivianite – PO43- pool in BDP aquifers.
Furthermore relatively high acetate fraction (Feacet ~ 0.02 – 0.37 mg kg-1 in Feox ~ 2.1 – 80%, Mnacet ~ 0.002 – 0.27 mg kg-1 in Mnox ~ 15.8 –97%, Pacet ~ 0.7 – 73.7 mg kg-1 in Pox ~ 70 %) confirms the possible dissolution of carbonate (siderire and rhodochrosite) as well as unstable phosphate mineral (vivianite) that has already been demonstrated by our speciation model during aqueous phase discussion. The study (hydrogeochemical and chemical partitioning modeling) further suggests that thegroundwater were supersaturated with respect to carbonate and phosphate mineralswhich acted as sinks for Fe/Mn/PO43-, and thereby, control their solubility in groundwater. Carbonate minerals were important components of mineralogy of the BDP sediments and dissolution/precipitation of As from such mineral phases controlsgroundwater chemistry. Therefore, reaction kinetics are important in understanding thelocal short range spatial variations. Moreover, correlation between Feox and Pox (r2 = 0.74, Figure 6a) indicates reductive dissolution of the amorphous Fe–oxides together with surface bound PO43-, whereas Feacet and PO43-acet fraction do not exhibit such relationship. These suggest that the Fe-oxides – PO43- pool is more significant thanvivianite – PO43- pool in BDP aquifers.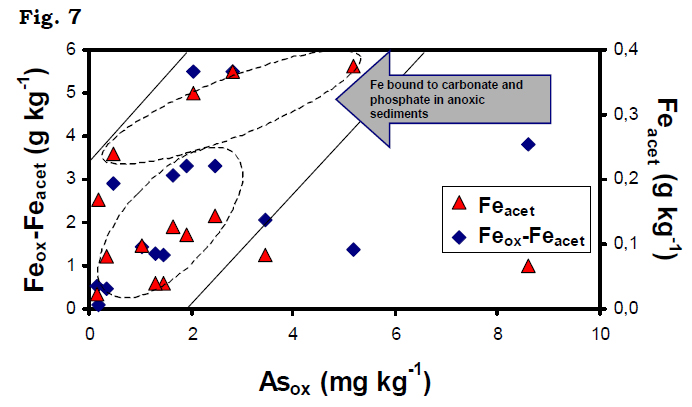 Fairly high correlation between Feox and Pox (r2 = 0.74; Figure 6a), indicates reductive dissolution of the amorphous Feoxides together with surface bound PO43- , particularly in the coarse sediments. However, Pacet fractions do not exhibit a significant correlation withFeacet (Figure 6b). Further, Pacet fractions are in the order of magnitude lower than the Pox fractions, which suggest that the pool of PO4 related to Fe-oxides is more significant than the pool of PO4 related to Pminerals like vivianite in the aquifers. Oxalate extractable Asox plotted against Feacet and Feox-Feacet as independent variables (Figure 7) reveals two distinct populations. The data sets plotted on this diagram, follow a linear trend for most of the analyzed sediments showing a clear association of Asox with the Fe-oxide phases (Feox-Feacet) in the coarse grained sediments. However, the reducing fine-grained aquifer sediments are characterized by high Feacet fractions plot separately. These results clearly demonstrate that siderite and vivianite are also present together with amorphous Fe-oxides with adsorbed As in the Holocene sedimentary aquifers.
Fairly high correlation between Feox and Pox (r2 = 0.74; Figure 6a), indicates reductive dissolution of the amorphous Feoxides together with surface bound PO43- , particularly in the coarse sediments. However, Pacet fractions do not exhibit a significant correlation withFeacet (Figure 6b). Further, Pacet fractions are in the order of magnitude lower than the Pox fractions, which suggest that the pool of PO4 related to Fe-oxides is more significant than the pool of PO4 related to Pminerals like vivianite in the aquifers. Oxalate extractable Asox plotted against Feacet and Feox-Feacet as independent variables (Figure 7) reveals two distinct populations. The data sets plotted on this diagram, follow a linear trend for most of the analyzed sediments showing a clear association of Asox with the Fe-oxide phases (Feox-Feacet) in the coarse grained sediments. However, the reducing fine-grained aquifer sediments are characterized by high Feacet fractions plot separately. These results clearly demonstrate that siderite and vivianite are also present together with amorphous Fe-oxides with adsorbed As in the Holocene sedimentary aquifers.Geochemistry and Arsenic affinity
The redox processes are important to understand the reduction reactions that occurswhen aquifers behave anoxic (Langmuir, 1997). Among the processes, ironoxides/hydroxides (FeIII/II system) redox chemistry is important since the system hasdirect impact on the mobility of As under anaerobic environment. The major pathway ofAs release in aquous phase is the reductive dissolution of the “arsenic traps” (mostlysedimentary Fe-oxides/hydroxides) under local reducing condition (redox traps, SOC).Arsenic is released to groundwater during reduction (partially and/or completely) ofthese arsenic traps (Fe/Mn/Al oxides/hydroxides) and the process is regulated by microbes (dissimilatory iron reducing bacteria, DIRB) where the oxidation of organicmatter supplies necessary energy to drive thermodynamically favored redox processesvia electron transfer reactions (Lovely and Chapelle, 1995). The redox ladder includesmultiple steps and begins with consumption of dissolved oxygen available in the subsurfacewater and an increase in dissolved bicarbonate ions due to the decomposition oforganic matter. Next is the de-nitrification process and aqueous nitrate decreasesrapidly with increase in dissolved bicarbonate ions. Sedimentary metaloxides/hydroxides are then reduced from insoluble phase to soluble phase [Mn (IV) (s)to Mn (II) (aq) and/or Fe (III) (s) to Fe (II) (aq)]. As a result, dissolved concentration ofredox sensitive species along with bicarbonate ions are increased in the system andsulphate reduction, methanogenesis and finally ammonia production are the sequentialsteps that are also important to understand redox processes. The speciation of redoxspecies (both solid and aqueous phase) and partitioning between sediment-waterinteraction are now important to understand the exact sequence of geochemicalprocesses that leads to the elevated redox species concentration in the groundwater(Sracek et al., 2004).
Our studies revealed that significant amounts of amorphous Fe-oxides/hydroxides,carbonates as well as SOC are present in the aquifer sands/silts. Arsenic is released togroundwater during reduction of these mineral phases (arsenic traps) under localreducing conditions (~1% SOC) and the process is regulated by microbes (mostly byDissimilator Iron Reducing Bacteria, DIRB) where oxidation of SOC supplies necessaryenergy to drive thermodynamically favoured redox processes. The sediment texture aswell as the presence and distribution of SOC vary in space. The high concentration ofredox sensitive species (As, Fe, Mn) along with high alkalinity and the absence ofdissolved oxygen and low to very low NO3- in the groundwater indicates the geochemical processes (dentrification → iron reduction) that controls the groundwater chemistry of the high As aquifers (hotspot areas). However, the vertical and lateral variation in redox species concentration (geographical distribution of As and public health issues) andtheir heterogeneity (at least in the hot spot areas) are difficult to explain by the processof iron reduction by subsurface organic rich sediment.
In BDP, the subsurface geochemical process is activated through the presence of highredox sensitive species. It is suggested that the respiration of organic carbon viacolloidal/pre-colloidal iron route plays an important role in As mobilization at best inshallow aquifers. However, mobilization may also be driven by reduction with organiccarbon via carbonate/silicates dissolution during the paucity of colloidal/pre-colloidaliron oxides. This can be a possible explanation to understand the heterogeneity of thehotspot areas where iron concentration is very low or insignificant and As concentrationis very high. Similar situation may also occur in deltaic/tidal/upland-inland basinareas where dissolved NH4+ and Ca2+ profiles follow the dissolved As concentration peak in natural groundwater and such observations are recently reported from various parts of BDP (Bhattacharya et al., 1997; Harvey et al., 2002; Akai et al., 2004; Sracek et al., 2004).
CONCLUSION
The present work suggests that the BDP groundwater is anoxic in nature and mostlycalcium bicarbonate type. Sedimentary iron [Fe(III)/(II)] is the dominant mineralconstituent that carry As might have deposited by the meandering river. Sedimentmineralogy and texture along with organic matter play crucial role in release of As ingroundwater. The possible geochemical pathways is Fe reduction at least in shallowaquifers. High redox sensitive species (As, Fe and Mn), high alkalinity and absence ofdissolved oxygen and nitrates further demonstrate the microbial mediated and thermodynamically favoured redox processes (denitrification iron-reduction). Aqueousspeciation of As reveals that both As(III) and As(V) concentrations are varying largelyand As(III) is significantly increasing in low lying areas. Stratigraphic distribution ofAs(III) and As(V) reveals that As(III) is more dominant in near-surface aquifers rich inorganic matter. Chemical partitioning further supports the presence of amorphous Feoxide together with surface bound phosphates in hotspot areas.
ACKNOWLEDGEMENTS
The principal author (BN) would like to acknowledge DAAD (Germany) for the fellowshipand opportunity to carry research work in Germany. One of the author (DC)acknowledges the funding agencies (RGNDWM/IFCPAR) to carry out the research workand acknowledges the Swedish and French partners for their active supports inanalysis and training of research scholars. We appreciate the critical comments ofGunnar Jacks to improve the manuscript. Another co-author (DS) likes to thankDepartment of Geological Sciences, J.U. for necessary infrastructural facilities to carryout the work.
REFERENCES
• Ahmed, K.M., Imam, M.B., Akhter, S.H., Hasan, M.A., Khan, A.A., 2001. In: Jacks,G., Bhattacharya, P., Khan, A.A. (Eds.), Groundwater Arsenic Contamination in theBengal Delta Plain of Bangladesh. Proc. KTH-Dhaka University Seminar. KTHSpecial Publication, TRITA-AMI Report 3084, pp. 97-108.
• Ahmed, K.M., Bhattacharya, P., Hasan, M.A., Akhter, S.H., Alam, S.M.M., Bhuyian,M.A.H., Imam, M.B., Khan, A.A., Sracek, O., 2004. Appl. Geochem. 19, 181-200.
• Akai, J., Izumi, K., Fukuhara, H., Masuda, H., Nakano, S., Yoshimura, T., Ohfuji,H., Hossain, M.A., Akai, K., 2004. Appl. Geochem. 19, 215-230.
• BGS and DPHE, 2001. BGS Technical Report WC/00/19 Vol 2 Final Report.
• Bhattacharya, P., Chatterjee, D., Jacks, G., 1997. J Wat. Res. Dev. 13, 79-92.
• Bhattacharya, P., Sracek, A., Jacks, G., 1998. Arsenic Crisis Information Center.http://bicn.com/acic/infobank/dch98-02/bp2.htm. (Accessed on January 20,2003).
• Bhattacharyya, R., 2001. Published doctoral thesis University of Kalyani, Kalyani,Nadia, West Bengal.
• Bhattacharya, P., Jacks, G., Jana, J., Sracek, A., Gustafsson, J.P., Chatterjee, D.,2001. In: Jacks, G., Bhattacharya, P., Khan, A.A. (Eds.), Groundwater ArsenicContamination in the Bengal Delta Plain of Bangladesh, KTH Special Publication.TRITA-AMI Report 3084, pp. 21-40.
• Bhattacharya, P., Frisbie, S.H., Smith, E., Naidu, R., Jacks, G., Sarkar, B., 2002a.In: Sarkar, B. (Eds.), Handbook of Heavy Metals in the Environment. Marcell DekkerInc. New York, pp. 147-215.
• Bhattacharya, P., Jacks, G., Ahmed, K.M., Khan, A.A., Routh, J., 2002b. Bull. Env.Cont. Toxicol. 69, 538-545.
• Bhattacharya, R., Jana, J., Nath, B., Sahu, S.J., Chatterjee, D., Jacks, G., 2003.Appl. Geochem. 18, 1435-1451.
• Brammer, H., 1996. The Geography of the Soils of Bangladesh University Press Ltd.,Dhaka.
• Chatterjee, D., Roy, R., Basu, B.B., 2004. A report of School of FundamentalResearch Calcutta.
• Dodd, J., Large, D.J., Fortey, N.J., Milodowski, A.E., Kemi, S., 2000. Env. Geochem.Health 22, 281-296.
• Goodbred, S.L., Kuehl, S.A., 2000. Sed. Geol. 133, 227-248.
• Guha Mazumder, D.N., De, B.K., Santra, A., Das Gupta, J., Ghose, N., Roy, B.K.,Ghosh, U.C., Saha, J., Chatterjee, A., Dutta, S., Haque, R., Smith, A.H.,Chakraborty, D., Angle, C.R., Centeno, J.A., 1999. In: Chappell, W.R., Abernathy,C.O., Calderon, R.L. (Eds.), Chapman and Hall, London, pp. 335 – 347.
• Harvey, C.F., Swartz, C.H., Badruzzaman, A.B.M., Keon-Blute, N., Yu, W., Ali, M.A.,Jay, J., Beckie, R., Nieden, V., Brabander, D., Oates, P.M., Ashfaque, K.N., Islam,S., Hemond, H.F., Ahmed, M.F., 2002. Science 298, 1602-1606.
• Karim, M.M., 2000. Wat. Res. 34, 304-310.
• Langmuir, D., 1997. Aqueous Environmental Geochemistry, Prentice-Hall.
• Lovely, D.R., Chapelle, F.H., 1995. Rev. Geophys. 33, 365.
• Meng, X.G., Wang, W., 1998. Third International Conference on Arsenic Exposureand Health Effects: Society of Environmental Geochemistry and Health, Universityof Colorado.
• Morgan, J.P., McIntire, W.G., 1959. Bull. Geol. Soc. Am. 70, 319-342.
• Mukherjee, A.B., Bhattacharya, P., 2001. Environ. Rev. 9, 189-220.
• Mukherjee, S. (1999). Remote sensing Applications in Applied Geosciences.Published by Manak Publications. New Delhi. ISBN 81-86562-69-9
• Nickson, R.T., McArthur, J.M., Ravenscroft, P., Burgess, W.G., Ahmed, K.M., 2000.Appl. Geochem. 15, 403-413.
• Parkhurst, D.L., 1995. Users guide to PHREEQC-A computer program forspeciation, reaction-path, advective-transport, and inverse geochemicalcalculations, U.S. Geological Survey Water-Resources Investigation Report 95-4227.
• PHED, 1993. National Drinking Water Mission project final report, Govt. of WestBengal, India.
• Saha, K.C., 1984. Indian J. Dermatol. 29, 37-46.
• Smedley, P.L., Kinniburgh, D.G., 2002. Appl. Geochem. 17, 517-568
• Sracek, A., Bhattacharya, P., Jacks, G., Chaterjee, D., Larsson, M., Liess, A., 2000.In: Ramanathan, A.L., Subramanian, V., Ramesh, R. (Eds.), Proc. InternationalSeminar on Applied Hydrogeochemistry, Annamalan University, Tamil Nadu, India,pp. 47-56.
• Uddin, A., Lundberg, N., 1998. Bull. Geol. Soc. Am. 110, 497-511.
• Umitsu, M., 1987. Geog. Rev. Japan (Ser B) 60, 164-178.
Debashis Chatterjee, Bibhash Nath, Joydeb Jana, Aishwarya Goswami, SudiptoChakraborty, Partha Mukherjee -Department of Chemistry, University of Kalyani, Kalyani, Nadia – 741235, West Bengal, India
Debasish Shome, Debatri Bagchi, Madhab Jyoti Sarkar, - Department of Geological Sciences, Jadavpur University, Calcutta – 700 032, India
Prosun Bhattacharya, Gunnar Jacks -Department of Land and Water Resources Engineering, Royal Institute of Technology, SE-100 44 STOCKHOLM, Sweden
Kazi Matin Ahmed - Department of Geology, University of Dhaka, Dhaka 1000, Bangladesh
Posted by













